Research
Background
Hearing and balance disorders affect millions worldwide, with nearly 1 in 8 people in the U.S. experiencing some form of hearing loss, and balance dysfunctions significantly impacting quality of life. The sensory cells of the inner ear - called hair cells - play a crucial role in detecting sound vibrations and head movements by converting mechanical stimuli into electrical signals for the brain to interpret. These hair cells are highly sensitive to damage and are not naturally regenerated in humans and other mammals, resulting in permanent inner ear dysfunction.
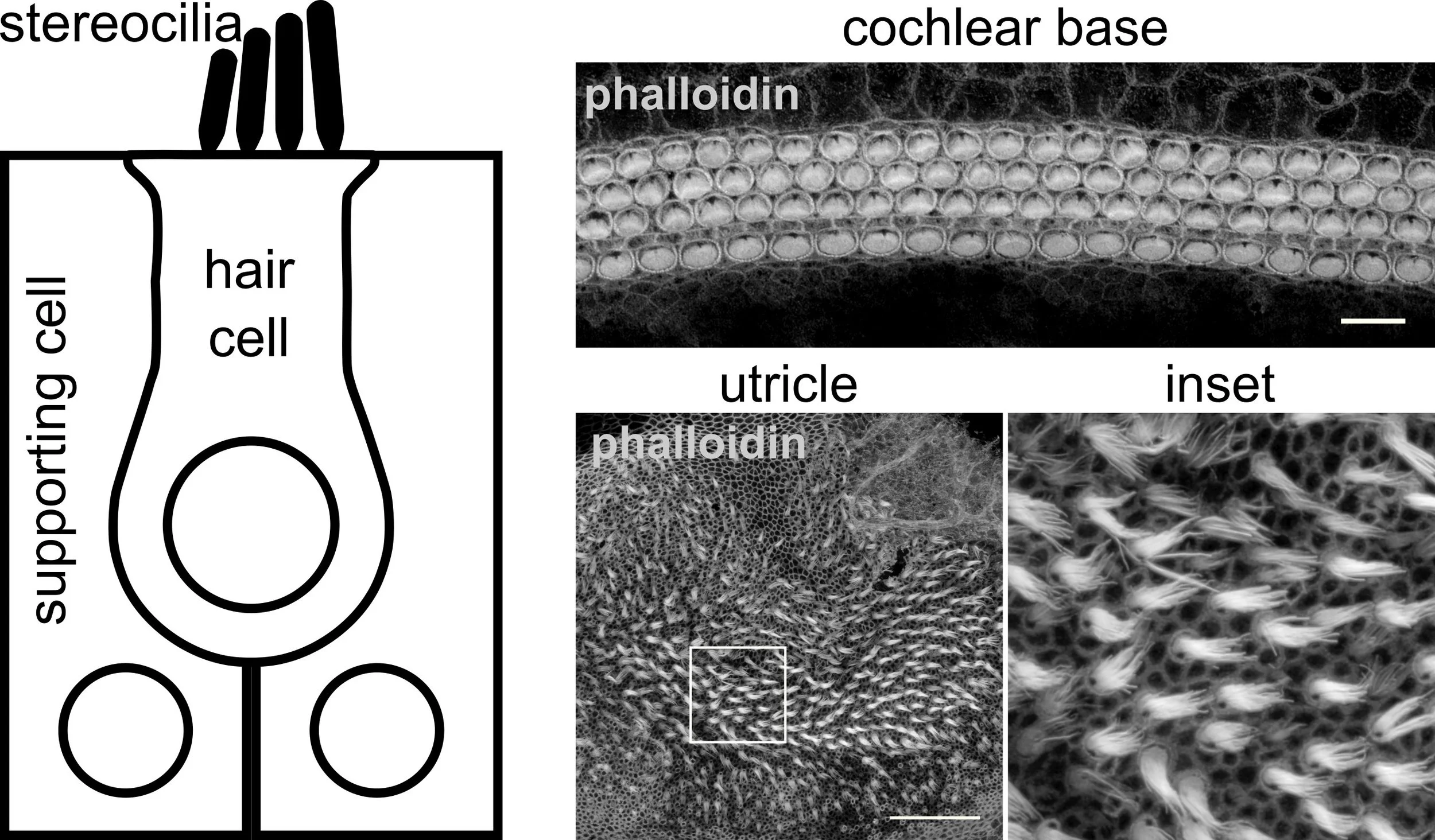
Inner ear sensory hair cells possess actin-based projections (called stereocilia) that detect vibrations from sound and movement. Actin can be stained with phalloidin, allowing us to visualize the stereocilia in the hearing and balance sensory epithelia of a newborn mouse (Matern et al., 2020).
Inner ear organoids - a tool for studying sensory cell development and regeneration
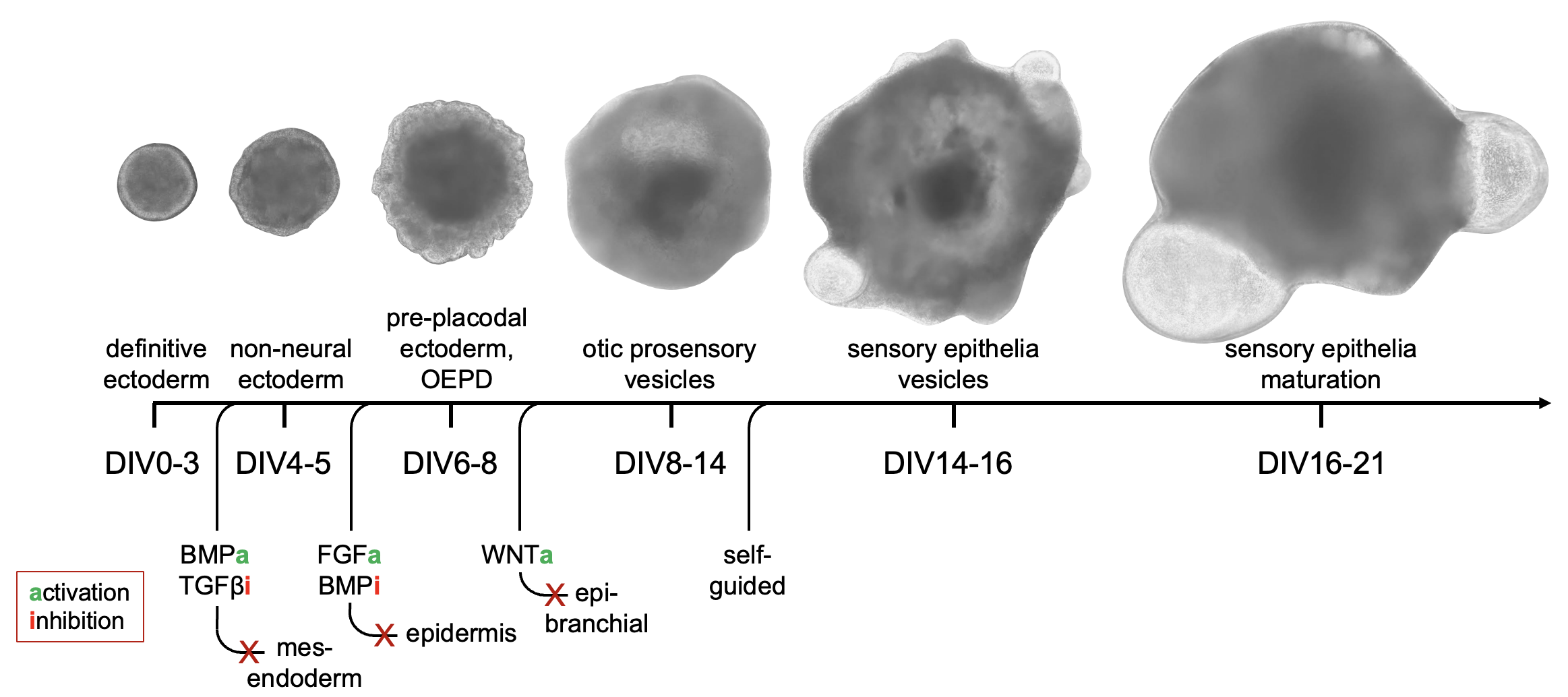
Our lab utilizes an organoid (or “organ-in-a-dish”) model to study how inner ear cells develop and respond to different genetic insults. This well-established protocol can produce inner ear sensory cells in as little as 2 weeks in culture.
Taking what we know about the initiation of inner ear development during embryogenesis, we can mimic those same signals to produce inner ear sensory epithelia in the lab.
Using scRNA-seq to study organoid supporting cells and hair cells
Our lab uses next generation sequencing technology and bioinformatics to study how organoid inner ear cells grow and respond to damage over time at the genetic level.
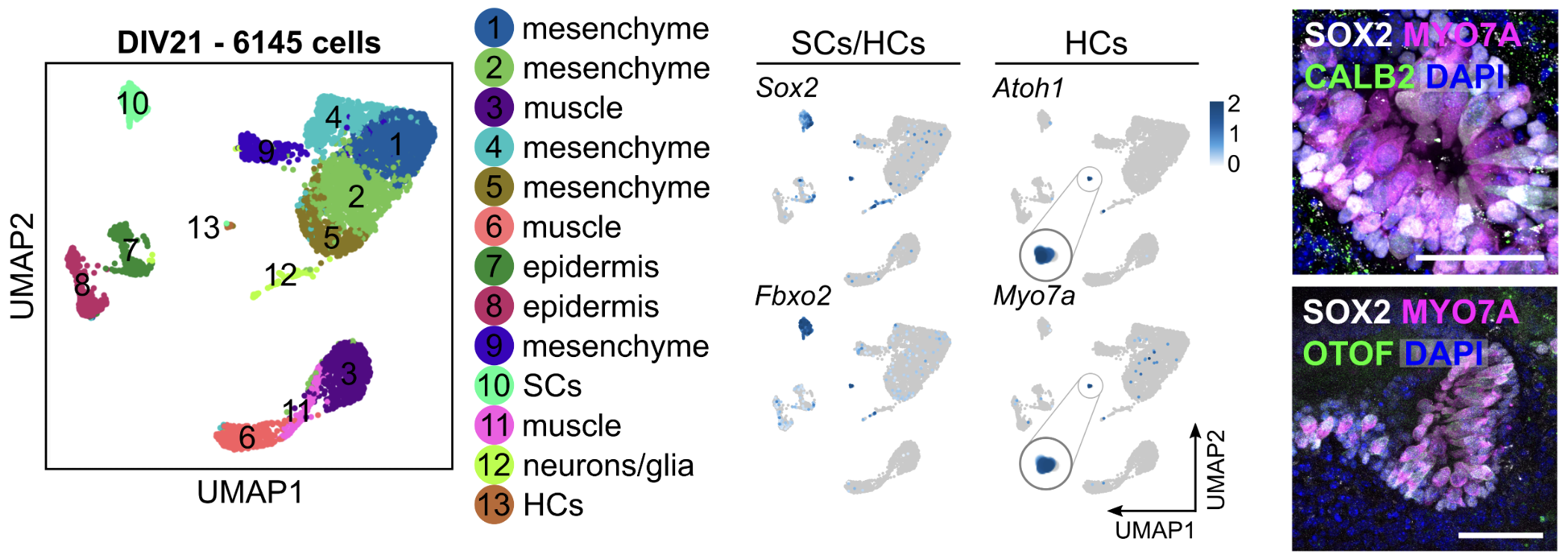
This UMAP plot represents single-cell RNA sequencing data from a 21 day old inner ear organoid. Each dot corresponds to an individual cell, which expresses distinct genes known as "marker genes." These marker genes help us to identify specific cell types of interest, such as hair cells (HCs) expressing Myo7a and supporting cells (SCs) expressing Sox2 (Matern et al., 2025).
Modulating gene expression with CRISPR
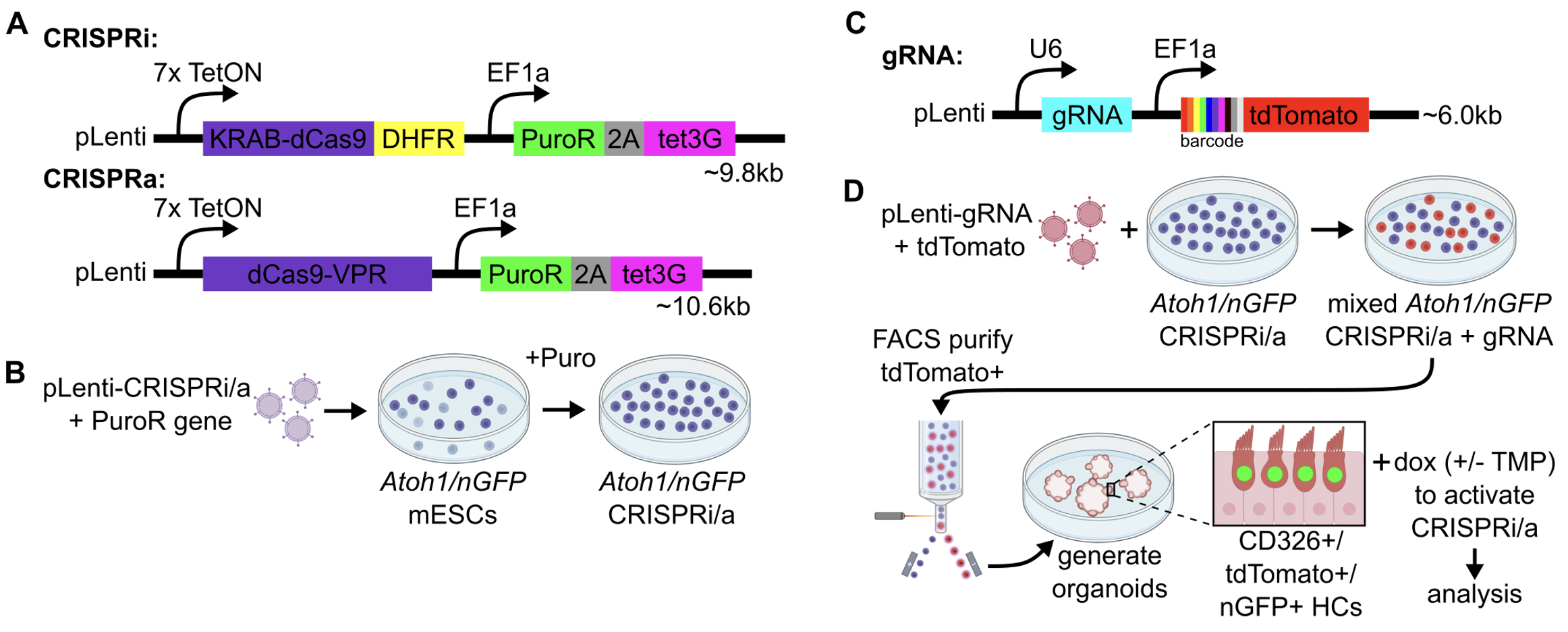
We use lentiviruses to generate new stem cell tools (called transgenics) that can help us manipulate gene expression in organoids. We are currently combining this approach with inducible CRISPR constructs to modulate gene expression in organoid-derived inner ear cells, helping us to better understand the roles that different genes play in development and to explore pathways for regeneration.
By swapping CRISPR targeting guide RNA sequences (C), we can target any gene in the genome and study how it may function in inner ear cells.
Interested in learning more?
Our ultimate goal is to better understand the genetics of hair cell development, so that we can regenerate hair cells in the damage inner ear and restore function. Contact us to see how you can get involved!